Spectrum Of Incandescent Light Bulb
Sources of Visible Light
Visible lite comprises only a tiny fraction of the unabridged electromagnetic radiation spectrum, yet information technology contains the only region of frequencies to which the rods and cones of the human eye will respond. The wavelengths that humans are typically able to visualize lie in a very narrow range betwixt approximately 400 and 700 nanometers. Humans can find and respond to stimuli created past visible light considering the eyes comprise specialized nervus endings that are sensitive to this range of frequencies. However, the remainder of the electromagnetic spectrum is invisible.
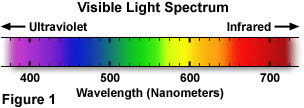
A wide diversity of sources are responsible for emission of electromagnetic radiation, and are by and large categorized according to the specific spectrum of wavelengths generated past the source. Relatively long radio waves are produced by electrical electric current flowing through huge broadcast antennas, while much shorter visible low-cal waves are produced by the energy land fluctuations of negatively charged electrons inside atoms. The shortest form of electromagnetic radiations, gamma waves, results from decay of nuclear components at the middle of the cantlet. The visible light that humans are able to see (the spectrum is illustrated in Effigy 1) is ordinarily a mixture of wavelengths whose varying limerick is a function of the light source.
In our everyday lives, we are bombarded by an enormous spectrum of electromagnetic radiations, but a portion of which we are able to actually "see" as visible light. When venturing outside, a vast majority of the calorie-free visible to humans is emitted from the dominicus, which also produces many other frequencies of radiation that practise not fall into the visible range. Inside, we are exposed to visible light that originates from artificial sources, primarily fluorescent and incandescent tungsten devices.
At night, natural light is produced by celestial bodies, such equally the moon, planets, and stars, in addition to the periodic Aurora Borealis (Northern Lights), and the occasional comet or meteor ("shooting star"). Other natural light sources include meteorological lightning, volcanoes, forest fires, plus some biochemical sources of visible light (bioluminescence). The biological light sources include the familiar lightning bugs ("fireflies") and more exotic glows from the ocean, including bioluminescent species of bacteria, algae, dinoflagellates, jellyfish, comb-jellies (ctenophores), and some species of fish.
Visible Light Wavelength and Perceived Colour
Table 1
Table one contains a listing of the credible color distribution perceived by humans for a number of narrow wavelength bands in the visible light spectrum. Relating specific colors to a region of wavelengths enables the differentiation betwixt different tones, hues, and shades. It is possible for many unlike spectral distributions to produce identical color sensations (a phenomenon known equally metamers). For example, a yellow color sensation may exist caused by a single wavelength of light, for instance 590 nanometers, or information technology may be the event of viewing two equal amounts of calorie-free having individual wavelengths, such every bit 580 and 600 nanometers. It is as well possible to view the colour yellow every bit a narrow distribution encompassing all wavelengths between 580 and 600 nanometers. With regards to the human visual system, the same argument holds for all colors in the visible spectrum. However, recent studies indicate that some species (most notably, birds) can discriminate between colors perceived as metamers by humans.
Incandescent Light Sources
Early humans were without a reliable source of light during the long nights, just they could occasionally find and collect burning forest from bush fires, and then go on the flames blazing in a campfire for a short period of time. As knowledge progressed, man discovered that sparks, and afterward burn, could be generated past hitting certain stones together (such as flint and fe pyrite) or past aggressively rubbing woods against woods. Once these techniques were mastered, man could produce burn whenever it was desired.
When a fire burns, chemic energy is released in the form of heat and light. The burning fuel, whether information technology is grass, woods, oil, or some other flammable material, emits gases that are heated past the enormous chemical free energy generated during combustion, making atoms in the gas glow or incandesce. Electrons within the gas atoms are promoted to college energy levels by the heat, and light is released in the form of photons when the electrons relax to their ground state. The color of a flame is an indication of the temperature and how much energy is being released. A dull xanthous flame is much cooler than a bright blueish flame, but even the coolest flame is still very hot (at least 350 degrees Celsius).
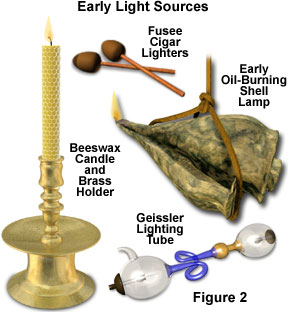
Although tar and rags were employed to produce early torches, the first applied step in controlling burn occurred when the oil lamp was invented. Early lamps over 15,000 years one-time (Figure 2) have been discovered, made from rocks and shells, which burned animal fat and plant oils. Before gas lighting was invented, in that location was a tremendous demand for creature oil. The principal source of this oil was the tallow produced by humid down fat tissues obtained from sea animals, such equally whales and seals. Oil lamps eventually evolved into candles that were formed past casting hardened tallow or beeswax, as illustrated in Effigy ii. Early candles generated quite a flake of smoke, just non much light. Somewhen, it was discovered that paraffin wax, when properly cast with an impregnated cloth wick, produced a relatively bright flame without a pregnant corporeality of smoke.
During the nineteenth century, natural gas lighting became widespread throughout many of the major towns and cities of Europe, Asia, and the United States. Early gaslights operated by producing a jet of burning gas (a quite dangerous situation), while afterwards models were fitted with a mantle, or fine internet of chemically treated fabric, which disperses the flame and emits a much brighter light.
Early microscopists relied on candles, oil lamps, and natural sunlight to provide illumination for the relatively crude optical systems in their microscopes. These primitive lite sources suffered from flickering, uneven illumination, glare, and often were a potential fire hazard. Today, incandescent high-intensity tungsten-based lamps are the primary calorie-free source utilized in modern microscopes and the majority of household lighting systems.
Presented in Figure 3 are spectral distribution curves demonstrating the relative amounts of free energy versus wavelength for several different sources of white light (comprised of a mixture containing all or well-nigh of the colors in the visible spectrum). The blood-red curve represents the relative free energy of tungsten low-cal over the entire visible spectrum. As is credible from examining the effigy, the energy of tungsten light increases as wavelength increases. This effect dramatically influences the average color temperature of the resultant light, peculiarly when it is compared to that of natural sunlight and fluorescent light (the mercury vapor lamp). The spectrum represented by a xanthous curve profiles the visible low-cal distribution from the natural sunlight spectrum sampled at noon. Under normal circumstances, sunlight contains the greatest amount of energy, merely the curves illustrated in Effigy iii accept all been normalized to the tungsten spectrum in lodge to ease comparing. The dark blue spectral bend is characteristic of a mercury arc lamp, and exhibits some notable differences from the tungsten and natural sunlight spectra. Several free energy peaks are present in the discharge arc lamp spectrum that occur a result of superposed individual line spectra originating from the mercury vapor.
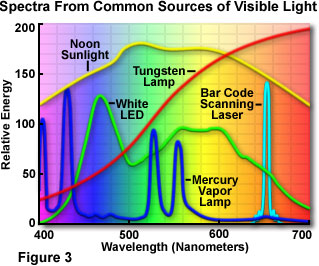
The visible light spectrum produced by a white light emitting diode (LED) is represented by the green curve in Effigy three. Light emitting diodes are inherently monochromatic devices, with the color existence determined by the ring gap between diverse semiconductor materials utilized in diode construction. Reddish, green, yellow, and blue diodes are common, and extensively employed as indicator lights for computers and other consumer electronics devices, such as radio tuners, television receiver receivers, compact disk players, videocassette recorders, and digital videodisk players. White light LEDs are fabricated from gallium nitride blueish diodes by blanket the semiconductor die with a phosphor material, which emits a broad range of visible wavelengths when excited by low-cal emitted from the blue diode. Laser spectra, whether derived from diodes or gas lasers, are characteristically very narrow, often comprising but one or a few specific wavelengths. An instance is illustrated in Figure 3 (the cyan curve) for a low-current semiconductor diode light amplification by stimulated emission of radiation that is useful for a variety of applications, including reading barcodes and tracking optical disk data.
Tungsten light sources are commonly termed incandescent, because they radiate light when heated by electrical energy. The filaments of modern light bulbs (or lamps) are generally equanimous of tungsten, a metal that is somewhat efficient at radiating light when resistively heated by an electrical electric current. Modern incandescent lamps descended from the carbon arc lamps invented by Sir Humphrey Davy, which produce light past a discharge arc formed between two carbon rods (or filament electrodes) when an electrical potential is placed across the electrodes. Ultimately, the carbon arc lamp gave mode to the first lamps that utilized carbon filaments contained in an evacuated drinking glass envelope. Tungsten filaments, pioneered in 1910 by William David Coolidge, evaporate much more than slowly than cotton fiber-derived carbon fibers when heated in the vacuum of a glass envelope. The filament acts as a unproblematic resistor, and emits a significant corporeality of light in improver to the heat generated past current period.
| Interactive Tutorial | | |
Tungsten incandescent lamps are thermal radiators that emit a continuous spectrum of light extending from virtually 300 nanometers, in the ultraviolet region, to about 1400 nanometers, in the near infrared region. Their design, construction, and operation are very simple, and a wide variety of these lamps accept been utilized as incandescent low-cal sources. Typical lamps consist of an sealed glass envelope (encounter Figure 4), evacuated or filled with an inert gas, and containing a tungsten wire filament that is energized past either direct or alternating current. The bulbs produce a tremendous amount of light and heat, only the lite accounts for just 5 to ten percent of their total energy output.
Tungsten lamps tend to suffer several drawbacks, such equally a decreased intensity with historic period and a blackening of the within envelope surface equally evaporated tungsten is slowly deposited onto the glass. The colour temperature and luminance of tungsten lamps vary with the applied voltage, but average values for color temperature range from about 2200 K to 3400 Thou. The surface temperature of an active tungsten filament is very loftier, typically averaging 2,550 degrees Celsius for a standard 100-watt commercial light bulb. In some cases, tungsten bulb envelopes are filled with the Noble gases krypton or xenon (inert fill gas) as an culling to creating a vacuum in order to protect the hot tungsten filament. These gases ameliorate the efficiency of incandescent lamps because they reduce the amount of evaporated tungsten that is deposited on the interior of the surrounding glass vessel.

Halogen bulbs, a high-performance version of the incandescent tungsten lamp, typically comprise traces of iodine or bromine in the fill gas, which return evaporated tungsten to the filament far more efficiently than lamps made with other gases. Tungsten-halogen lamps, first developed by General Electric in the 1950s for lighting the tips of supersonic jet wings, are capable of producing very uniform bright light throughout the bulb lifetime. In addition, halogen lamps are much smaller and more efficient than tungsten lamps of comparable intensity. The lifetime of a tungsten-halogen seedling can be as much as 10 years under the most platonic conditions.
The filaments of tungsten-halogen lamps are often very meaty spiral assemblies mounted in a borosilicate-halide drinking glass (oftentimes termed fused quartz) envelope. High operating temperatures restrict the employ of tungsten-halogen bulbs to well-ventilated lamphouses with fan-shaped heat sinks to eliminate the tremendous amount of heat generated past these bulbs. Many household lamps are equipped to operate with 300-500 watt tungsten-halogen lamps, and produce a significant amount low-cal that fills a room much better than their weaker-emitting tungsten counterparts. When coupled with fiber optic light pipes and absorption or dichromatic filters, tungsten-element of group vii lamphouses provide high intensity illumination for a wide multifariousness of optical microscopy applications, only as a major disadvantage, produce significant amounts of infrared light in the form of radiant heat that tin can easily degrade the specimen.
Fluorescent Lite Sources
There are a broad variety of not-incandescent visible calorie-free sources that are employed for indoor and outdoor lighting, in addition to having important applications in optical microscopy. Most of these lite sources are based on electric discharge through a gas such as mercury, or the Noble gases neon, argon, and xenon. The generation of visible low-cal in gas discharge lamps relies on collisions between atoms and ions in the gas with an electrical current that is passed betwixt a pair of electrodes placed at the ends of the bulb envelope.
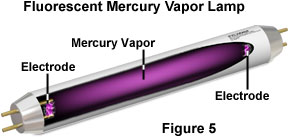
The drinking glass tube of a common fluorescent lamp is coated with phosphor on the inside surface of the glass, and the tube is filled with mercury vapor at very depression pressure level (see Effigy v). An electric current is applied between the electrodes at the ends of the tube, producing a stream of electrons that flow from one electrode to the other. When electrons from the stream collide with mercury atoms, they excite electrons inside the atoms to a higher energy state. This energy is released in the form of ultraviolet radiation when electrons in the mercury atoms return to the ground land. The ultraviolet radiation afterward energizes the internal phosphor coating, causing it to emit the bright white lite that we discover from fluorescent lights. Fluorescent lamps are virtually ii to four times more efficient at emitting visible lite, produce less waste heat, and typically terminal ten to twenty times longer than incandescent lamps.
A unique feature of fluorescent light sources is that they generate a series of wavelengths that are often concentrated into narrow bands termed line spectra. Equally a consequence, these sources do not produce the continuous spectrum of illumination that is characteristic of incandescent sources. A skillful case of a (well-nigh exclusively) single wavelength source of non-incandescent visible calorie-free is the sodium-vapor lamps commonly employed in street lighting. These lamps emit a very intense yellow calorie-free, with over 95 per centum of the emission existence composed of 589-nanometer light and nigh no other wavelengths nowadays in the output. It is possible to design gas-belch lamps that volition emit a near continuous spectrum in addition to the line spectra inherent in near of these lamps. The most common technique is to coat the inside surface of the tube with phosphor particles, which will absorb radiations emitted by the glowing gas and convert it into a wide spectrum of visible light ranging from blue to red.
Nether normal circumstances, most individuals are not able to discern the difference betwixt a line spectrum and a spectrum of continuous wavelengths. However, some objects reflect unusual colors in light from a discontinuous source, specially under fluorescent lighting. This is why habiliment, or other highly colored items, purchased in a shop illuminated past fluorescent light ofttimes appears a slightly different colour nether natural sunlight or continuous tungsten illumination.
| Interactive Tutorial | | |
In reflected calorie-free stereomicroscopy, peculiarly when examining heat-sensitive specimens, fluorescent lamps are favored over tungsten lamps considering of their high efficiency and low heat output. Modern fluorescent lamps can be configured for linear tube or ring illuminators to provide the microscopist with intense, lengthened calorie-free. This source of bogus white light rivals sunlight (without the accompanying estrus) in color temperature, and eliminates the flicker characteristics typical of consumer-class fluorescent tubes. In comparison to tungsten, tungsten-halogen, or arc lamps, fluorescent-lamp microscope illuminators can provide relatively long periods (approximately 7,000 hours) of high quality service. Every bit a diffuse light source, fluorescent lamps produce an evenly illuminated field of view without annoying hot spots or glare. Newer cold cathode illumination technology shows promise equally a specialized calorie-free source in optical microscopy, specially for brusk-lived events enhanced by fluorescence excitation, and for applications where waste estrus or warm-up fourth dimension in a light source may interfere with the specimen or the event being observed.
A specialized method for photographing moving specimens, particularly useful in darkfield microscopy illumination, has been devised using electronic photography flash systems. Electronic wink units operate through ionization in a xenon gas-filled drinking glass envelope driven by the discharge of a large capacitor. The short-lived, high-voltage pulse from a transformer induces the xenon gas to ionize, assuasive the capacitor to belch through the at present-conductive gas. A sudden outburst of bright light is emitted, after which the xenon gas rapidly returns to a non-conductive state, and the capacitor recharges. Flash tubes provide five,500 M illumination in an instantaneous burst that can capture a significant amount of object detail for spectacular results in photography, digital imaging, and photomicrography.
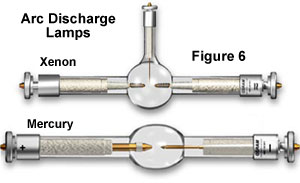
Arc discharge lamps, filled with gases such as mercury vapor and xenon, are favored sources of illumination for some specialized forms of fluorescence microscopy. A typical arc lamp is x-100 times brighter than tungsten-based counterparts and can provide vivid monochromatic illumination when combined with specially coated dichromatic interference filters. Unlike tungsten and tungsten-halogen lamps, arc lamps practise not contain a filament, only rather, depend on ionization of the gaseous vapor though a high-energy arc discharge betwixt two electrodes to produce their intense light. In general, arc lamps have an boilerplate lifetime of virtually 100-200 hours, and most external power supplies are equipped with a timer that enables the microscopist to monitor how much time has elapsed. Mercury arc lamps (often referred to as burners; run across the mercury and xenon lamps illustrated in Effigy 6) range in power from 50 to 200 watts and usually consist of ii electrodes sealed nether high mercury vapor pressure in a quartz glass envelope.
Mercury and xenon arc lamps do non provide even illumination intensity across the entire wavelength spectrum from virtually ultraviolet to infrared. Much of the intensity of the mercury arc lamp is expended in the near-ultraviolet and blueish spectrum, with most of the high-intensity peaks occurring in the 300-450 nanometer range, except for a few higher-wavelength peaks in the light-green spectral region. In dissimilarity, xenon arc lamps take a broader and more than even intensity output across the visible spectrum, and do not exhibit the very high-spectral-intensity peaks that are characteristic of mercury lamps. Xenon lamps are deficient in the ultraviolet, even so, and expend a big proportion of their intensity in the infrared, requiring care in control and elimination of excess heat when these lamps are employed.
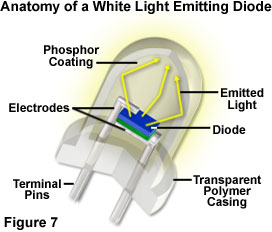
The era of utilizing light emitting diodes as a practical source of illumination has arrived with the twenty-first century, and the diode is an ideal complement to the spousal relationship of semiconductor technology and optical microscopy. The relatively low power consumption (1 to iii volts at 10 to 100 milliamperes), and long working life of light emitting diodes, renders these devices perfect light sources when low to medium intensity levels of white light are required. Microscopes connected to computers interfaced through a universal serial bus (USB) port, or powered by batteries, tin utilise the LED equally a small-scale, depression-rut, low-power, and depression-cost internal light source for visual observation and digital image capture. Several didactics and entry-level inquiry microscopes currently employ an internal, high-intensity white lite emitting diode that serves as the master lite source.
Although the epoxy envelope light project characteristics are all the same being explored, lite emitting diodes are currently being tested and marketed in a wide variety of applications, such as traffic signals, signs, flashlights, and external band-style illuminators for microscopy. The light produced past white LEDs has a colour temperature spectrum like to that of a mercury vapor lamp, which is in the daylight illumination category. Examining the white LED emission spectrum presented in Figure 3, the manual peak at 460 nanometers is due to blue lite emitted past the gallium nitride diode semiconductor, while the broad high-transmission range positioned between 550 and 650 nanometers results from secondary low-cal emitted past a phosphor coating inside the polymer jacket. The combination of wavelengths produces "white" light having a relatively high color temperature, which is a suitable wavelength range for imaging and observation in optical microscopy.
Laser Light Sources
Some other source of visible light that is becoming increasingly more than of import in our everyday lives is laser illumination. The acronym LASER is an abbreviation for 50ight Amplification past the Stimulated Eastmission of Radiation. Among the unique features of lasers is that they emit a continuous beam of light composed of a single discrete wavelength (or sometimes several wavelengths) that exits the device in a unmarried, aligned stage, commonly termed coherent light. The wavelength of light emitted by a laser depends upon the material from which the laser crystal, diode, or gas is equanimous. Lasers are produced in a variety of shapes and sizes, ranging from tiny diode lasers pocket-size enough to fit through the middle of a needle, to huge armed services and enquiry-grade instruments that fill an entire building.
Lasers are used as light sources in a number of applications ranging from compact disk readers to measuring tools and surgical instruments. The familiar red light of the helium-neon (often abbreviated He-Ne) laser scans consumer purchases by lighting optical bar codes, but as well plays a disquisitional part in many light amplification by stimulated emission of radiation scanning confocal microscopy systems. The application of lasers in optical microscopy is also growing in importance, both as a sole light source, and in combination with fluorescent and/or incandescent light sources. Despite the relatively loftier cost, lasers find peculiarly wide application in fluorescence, monochromatic brightfield, and in the apace growing fields of light amplification by stimulated emission of radiation scanning confocal, full internal reflection, fluorescence resonance energy transfer, and multi-photon microscopy.
| Interactive Tutorial | | |
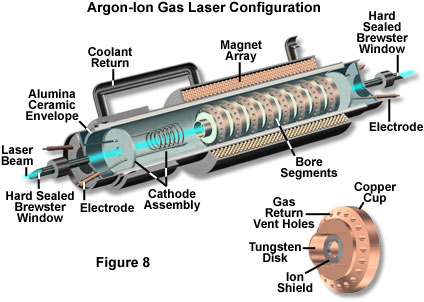
Argon-ion lasers (Figure 8) produce powerful spectral emissions at 488 and 514 nanometers, while krypton gas lasers showroom large peaks at wavelengths of 647.1 and 752.5 nanometers. Both of these lasers are often utilized every bit excitation sources in laser scanning confocal microscopy. Titanium-doped sapphire crystal mode-locked pulsed lasers are used as sources for multiphoton excitation due to their loftier summit intensity, only they also feature low average power and short duty cycles. As preferred light sources for multiphoton microscopy, pulsed lasers are considerably more expensive and difficult to operate than the small, air-cooled lasers employed in confocal microscopy.
Newer laser technology features semiconductor-based laser diodes and single on-chip lasers that reduce the size and power requirements for light sources. Light amplification by stimulated emission of radiation diodes, such as neodymium:yttrium lithium fluoride (Nd:YLF) and neodymium:yttrium vanadate (Nd:YVO(4)), typically are much faster in response than LEDs, only are besides relatively small and crave lilliputian power. Disadvantages of using lasers in microscopy include additional costs for the low-cal source, the risk of expensive impairment to optics, increased costs associated with lens and mirror coatings, destruction of specimens, and potential retinal damage to the microscopist if rubber handling and operating techniques are ignored.
From this discussion, it is apparent that although there are a broad diversity of available illumination sources, we generally rely on only a few throughout our everyday lives. During daylight hours the sun serves every bit our main source of illumination outdoors, while nosotros by and large rely on fluorescent and tungsten lighting while indoors and during the evening hours. As discussed higher up, these three principal lighting sources all have different backdrop and spectral characteristics, but their maximum intensities all fall within the visible calorie-free range. The man brain adjusts automatically to the unlike lite sources, and we translate the colors of almost objects around us as hardly changing when they are viewed under differing weather condition of illumination.
Contributing Authors
Kenneth R. Jump - Scientific Consultant, Lusby, Maryland, 20657.
Michael Due west. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State Academy, Tallahassee, Florida, 32310.
Back TO SOURCES OF VISIBLE LIGHT
Back TO LIGHT AND Colour
Questions or comments? Transport us an email.
© 1998-2022 by Michael W. Davidson and The Florida Land University. All Rights Reserved. No images, graphics, scripts, or applets may be reproduced or used in any style without permission from the copyright holders. Employ of this website means you agree to all of the Legal Terms and Weather set along by the owners.
This website is maintained past our
Graphics & Web Programming Team
in collaboration with Optical Microscopy at the
National High Magnetic Field Laboratory.
Concluding modification: Friday, Sep 07, 2018 at 03:48 PM
Access Count Since July one, 1998: 215327
For more than data on microscope manufacturers,
use the buttons below to navigate to their websites:
Spectrum Of Incandescent Light Bulb,
Source: https://micro.magnet.fsu.edu/primer/lightandcolor/lightsourcesintro.html
Posted by: hughesbegadd.blogspot.com


0 Response to "Spectrum Of Incandescent Light Bulb"
Post a Comment