Most Common Isotope Of Carbon
3.5: Isotopes
- Page ID
- 85148
Learning Objectives
- Calculate subatomic particles (protons, neutrons, and electrons) for whatever element by looking at the periodic table.
- Calculate subatomic particles (protons, neutrons, and electrons) for any element past looking at symbol-mass and A/Z format.
- Sympathise how isotopes differ in particles and mass.
- Identify the most abundant isotope when given specific values.
- Calculate the atomic mass of an element from the masses and relative percentages of the isotopes of the chemical element.
- Define LEU/HEU with percentages and applications.
Isotopes are atoms of the same chemical element that contain dissimilar numbers of neutrons. For these species, the number of electrons and protons remains constant. This divergence in neutron corporeality affects the atomic mass (A) but not the atomic number (Z). In a chemical laboratory, isotopes of an element appear and react the aforementioned. For this reason, information technology is difficult to distinguish between an cantlet'due south isotopes. In contrast, nuclear scientists can identify and dissever different types of diminutive nuclei (Figure \(\PageIndex{1}\)). The engineering required for this process is more sophisticated than what could be found in a typical chemic laboratory.
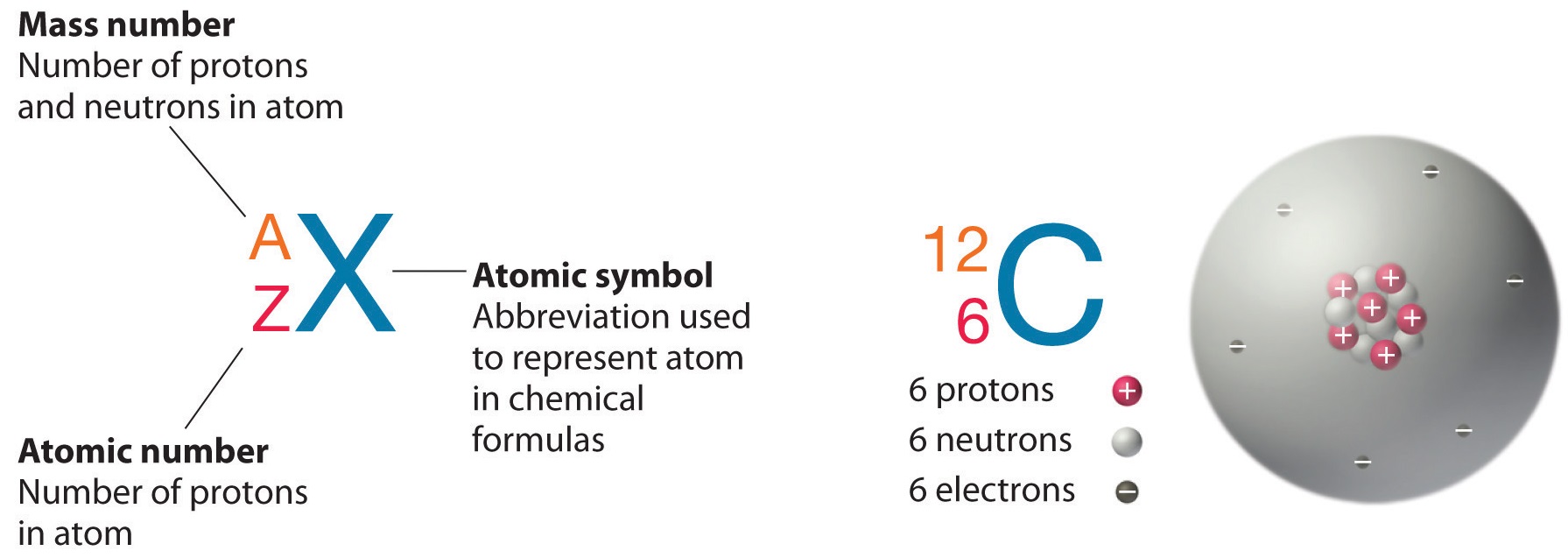
The element carbon (\(\ce{C}\)) has an diminutive number of 6, which means that all neutral carbon atoms incorporate 6 protons and 6 electrons. In a typical sample of carbon-containing material, 98.89% of the carbon atoms besides contain half-dozen neutrons, then each has a mass number of 12. An isotope of any chemical element tin be uniquely represented as \({}_Z^{A}Ten\)where X is the atomic symbol of the element. The isotope of carbon that has 6 neutrons is therefore \(\ce{_6^{12}C}\) The subscript indicating the diminutive number is actually redundant because the atomic symbol already uniquely specifies Z. Consequently, it is more often written as \(\ce{^{12}C}\), which is read equally "carbon-12." Nevertheless, the value of \(Z\) is commonly included in the note for nuclear reactions considering these reactions involve changes in \(Z\).
About elements on the periodic tabular array have at least two stable isotopes. For example, in improver to \(\ce{^{12}C}\), a typical sample of carbon contains 1.11% \(\ce{_6^{13}C}\), with 7 neutrons and 6 protons, and a trace of \(\ce{_6^{14}C}\), with 8 neutrons and vi protons. The nucleus of \(\ce{_6^{14}C}\) is not stable, even so, but undergoes a slow radioactive decay that is the basis of the carbon-14 dating technique used in archaeology. Many elements other than carbon accept more than one stable isotope; tin, for example, has 10 isotopes. There are about twenty elements that be in only ane isotopic course (sodium and fluorine are examples of these). Nearly scientists cannot tell you how many isotopic forms exist unless they consult an isotopic table.
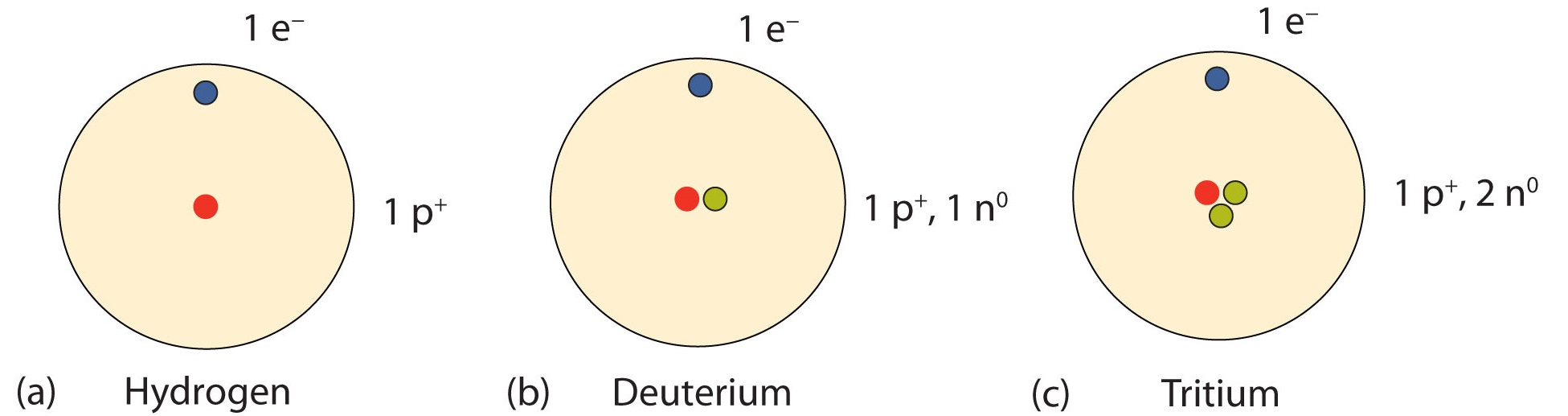
An important series of isotopes is plant with hydrogen atoms. Most hydrogen atoms take a nucleus with only a single proton. Well-nigh 1 in 10,000 hydrogen nuclei, however, also has a neutron; this particular isotope is called deuterium. An extremely rare hydrogen isotope, tritium, has ane proton and 2 neutrons in its nucleus. Figure \(\PageIndex{2}\) compares the 3 isotopes of hydrogen.
There are currently over 3,500 isotopes known for all the elements. When scientists discuss individual isotopes, they need an efficient fashion to specify the number of neutrons in whatever particular nucleus. A/Z and symbol-mass formats (refer to Department three.4) can be used to display periodic table information. When viewing either of these two notations, isotopic differences tin can be obtained.
The discovery of isotopes required a small change in Dalton's atomic theory. Dalton idea that all atoms of the aforementioned chemical element were exactly the same.
Expect at the A/Z formats for the three isotopes of hydrogen in Tabular array \(\PageIndex{ane}\). Notation how the atomic number (bottom value) remains the same while the atomic masses (elevation number) are varied. All isotopes of a particular element will vary in neutrons and mass. This variance in mass volition be visible in the symbol-mass format of aforementioned isotopes as well.
| Common Name | A/Z formats | symbol-mass format | Expanded Name |
|---|---|---|---|
| Hydrogen | \(\mathrm{^{1}_{1}H}\) | \(\text{H-1}\) | hydrogen-1 |
| Deuterium | \(\mathrm{^{two}_{1}H}\) | \(\text{H-ii}\) | hydrogen-2 |
| Tritium | \(\mathrm{^{3}_{1}H}\) | \(\text{H-3}\) | hydrogen 3 |
Using a periodic table, A/Z or symbol-mass formats can be utilized to decide the number of subatomic particles (protons, neutrons, and electrons) independent inside an isotope. When given either format, these mass values should exist used to calculate the number of neutrons in the nucleus.
Exercise \(\PageIndex{1}\)
How many neutrons are in each atom?
- \(\ce{^{36}_{17}Cl}\)
- \(\ce{^{58}_{26}Fe}\)
- \(\ce{C}\)-14
Solution
- In \(\mathrm{^{36}_{17}Cl}\) there are nineteen neutrons in this nucleus. To notice this value, subtract A-Z or 36 − 17.
- In \(\mathrm{^{58}_{26}Atomic number 26}\) there are 32 neutrons in this nucleus. Again, subtract A-Z or 58-26.
- In this example, the C-14 represents symbol-mass format. One time the atomic number is located (look at periodic table), subtract 14-6. The final answer will be eight neutrons.
Practice \(\PageIndex{2}\)
For the species below, translate the A/Z format to symbol-mass. And then, calculate the number of subatomics for each atom.
- \(\ce{^{197}_{79}Au}\)
- \(\ce{^{23}_{11}Na}\)
- \(\ce{^{239}_{94}Pu}\)
- Respond a
-
Au-197 contains 79 protons, 79 electrons, and 118 neutrons.
- Respond b
-
Na-23 contains 11 protons, 11 electrons, and 12 neutrons.
- Respond c
-
Pu-239 contains 94 protons, 94 electrons, and 145 neutrons.
Computing Atomic Mass
The diminutive mass of an element is the weighted mass of all the naturally presented isotopes. On the periodic table, it is causeless that this mass has units of amu (diminutive mass unit) which can exist abbreviated past using the alphabetic character u. To decide the virtually arable isotopic form of an element, compare given isotopes to the weighted average on the periodic table. For case, the three hydrogen isotopes (shown in a higher place) are H-1, H-2, and H-three. The atomic mass or weighted average of hydrogen is around 1.008 amu ( look once more at the periodic table). Of the three hydrogen isotopes, H-1 is closest in mass to the weighted average; therefore, it is the most abundant. The other 2 isotopes of hydrogen are rare but are very heady in the world of nuclear science.
Instance \(\PageIndex{two}\)
Identify the true statements:
- Al-27 is more arable than Al-25.
- An appropriate isotope of bromine could be Br-35.
- A chemist could hands distinguish between Cs-132 and Cs-133 by noting chemical and physical properties.
- Virtually scientists know that calcium has 24 isotopes.
- Respond a
-
This statement is true. Aluminum with a mass of 27 is closest to the mass on the periodic tabular array. Information technology would exist more than abundant than Al-25.
- Answer b
-
This statement is false. An appropriate mass number of an isotope of bromine would be in around 80 amu (atomic mass units), not 35 (which is the atomic number).
- Answer c
-
Chemists cannot distinguish between isotopes by looking at various properties. Most isotopes have like solubilities, densities, and colors.
- Answer d
-
Unless a scientist works heavily with a detail atom, he or she is non aware of the many forms that cannot exist. They would need a reference guide to know how many natural and artificial isotopes exist for a particular element.
You tin can calculate the atomic mass (or average mass) of an element provided you know the relative abundances (the fraction of an element that is a given isotope), the element's naturally occurring isotopes, and the masses of those different isotopes. Nosotros tin can calculate this by the following equation:
\[\text{Diminutive mass} = \left( \%_1 \right) \left( \text{mass}_1 \right) + \left( \%_2 \right) \left( \text{mass}_2 \right) + \cdots \characterization{eq1}\]
To confirm your answer, compare the calculated value to the weighted mass displayed on the periodic tabular array.
An element's diminutive mass can be calculated provided the relative abundances of the chemical element's naturally occurring isotopes and the masses of those isotopes are known. If all the abundances are non provided, it is safe to assume that all numbers should add up to 100%.
Instance \(\PageIndex{3}\): Diminutive mass of Boron
Boron has 2 naturally occurring isotopes. In a sample of boron, \(twenty\%\) of the atoms are \(\text{B-ten}\), which is an isotope of boron with 5 neutrons and mass of \(10 \: \text{amu}\). The other \(lxxx\%\) of the atoms are \(\text{B-11}\), which is an isotope of boron with 6 neutrons and a mass of \(eleven \: \text{amu}\). What is the diminutive mass of boron?
Solution
Boron has 2 isotopes. We will use the Equation \ref{eq1}:
\[\text{Atomic mass} = \left( \%_1 \right) \left( \text{mass}_1 \right) + \left( \%_2 \right) \left( \text{mass}_2 \right) + \cdots \nonumber\]
Substitute these into the equation, and we get:
\[\begin{align} \text{Atomic mass} &= \left( 0.20 \right) \left( 10 \correct) + \left( 0.80 \right) \left( eleven \right) \nonumber \\ &= 10.8 \: \text{amu}\nonumber \terminate{align}\nonumber \]
The mass of an boilerplate boron atom, and thus boron'southward atomic mass, is \(10.viii \: \text{amu}\).
Example \(\PageIndex{4}\): Atomic mass of Neon
Neon has three naturally occurring isotopes. In a sample of neon, \(90.92\%\) of the atoms are \(\ce{Ne}\)-20, which is an isotope of neon with 10 neutrons and a mass of \(19.99 \: \text{amu}\). Another \(0.3\%\) of the atoms are \(\ce{Ne}\)-21, which is an isotope of neon with xi neutrons and a mass of \(20.99 \: \text{amu}\). The concluding \(viii.85\%\) of the atoms are \(\ce{Ne}\)-22, which is an isotope of neon with 12 neutrons and a mass of \(21.99 \: \text{amu}\). What is the atomic mass of neon?
Solution
Neon has 3 isotopes. We will utilize the equation:
\[\text{Atomic mass} = \left( \%_1 \right) \left( \text{mass}_1 \right) + \left( \%_2 \correct) \left( \text{mass}_2 \right) + \cdots \nonumber\]
Substitute these into the equation, and we get:
\[\brainstorm{marshal*} \text{Atomic mass} &= \left( 0.9092 \correct) \left( xix.99 \right) + \left( 0.003 \right) \left( 20.99 \right) + \left( 0.0885 \right) \left( 21.99 \right) \\ &= 20.xviii \: \text{amu} \end{marshal*}\]
The mass of an boilerplate neon atom is \(20.eighteen \: \text{amu}\)
Applications of Isotopes
During the Manhattan project, the bulk of federal funding dedicated the separation of uranium isotopes. The two most common isotopes of uranium are U-238 and U-235. Well-nigh 99.iii% of uranium is of the U-238 variety, this course is not fissionable and will not piece of work in a nuclear weapon or reaction. The remaining .vii% is U-235 which is fissionable but first had to be separated from U-238. This separation procedure is called enrichment. During Globe State of war II, a nuclear facility was built in Oak Ridge, Tennessee to attain this projection. At the time, the enrichment process only produced enough U-235 for one nuclear weapon. This fuel was placed inside the smaller of the 2 atomic bombs (Little Male child) dropped over Japan.

Uranium is a natural chemical element that can exist constitute in several different countries. Countries that do not have natural uranium supplies would need to obtain it from one of the countries below. About nuclear reactors that provide energy rely on U-235 as a source of fuel. Fortunately, reactors only demand ii-5% U-235 for the production of megawatts or even gigawatts of power. If the purification process exceeds this level, then it is probable a state is focusing on making nuclear weapons. For example, Manhattan Projection scientists enriched U-235 upwardly to xc% in guild to produce the Little Male child weapon.
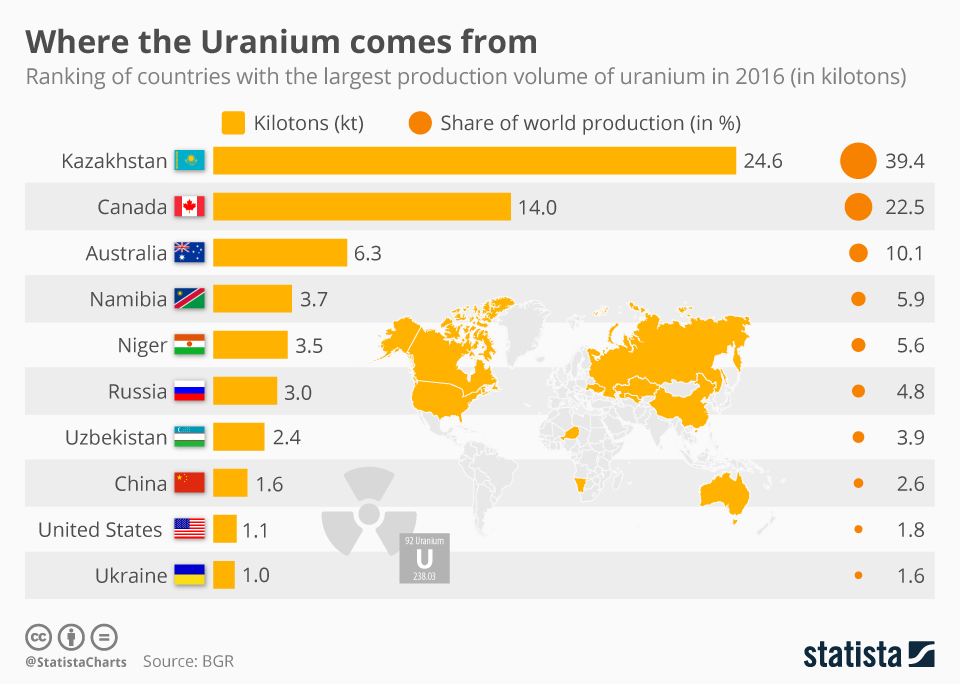
Abbreviations like HEU (highly enriched uranium) and LEU (low-enriched uranium) are used oft by nuclear scientists and groups. HEU is defined every bit being over 20% pure U-235 and would not be used in most commercial nuclear reactors. This type of material is used to fuel larger submarines and shipping carriers. If the purification of U-235 reaches xc%, and so the HEU is further classified as existence weapons-class material. This type of U-235 could exist used to make a nuclear weapon (fission or even fusion-based). As for LEU, its U-235 level would be below this 20% mark. LEU would be used for commercial nuclear reactors and smaller, nuclear-powered submarines. LEU is not pure enough to be used in a conventional nuclear weapon just could be used in a muddied flop. This blazon of weapon uses conventional explosives like dynamite to spread nuclear material. Different a nuclear weapon, dirty bombs are not powerful enough to affect big groups of buildings or people. Unfortunately, the spread of nuclear material would crusade massive anarchy for a community and would result in casualties.
Need More Practice?
- Turn to Section 3.E of this OER and work issues #three, #vi, #7, and #10.
Most Common Isotope Of Carbon,
Source: https://chem.libretexts.org/Courses/Furman_University/CHM101:_Chemistry_and_Global_Awareness_%28Gordon%29/03:_Atoms_and_the_Periodic_Table/3.05:_Isotopes
Posted by: hughesbegadd.blogspot.com


0 Response to "Most Common Isotope Of Carbon"
Post a Comment