Which Two Of The Following Reduce The Background Noise In Magneto- Medicine?
| Magnetoencephalography | |
|---|---|
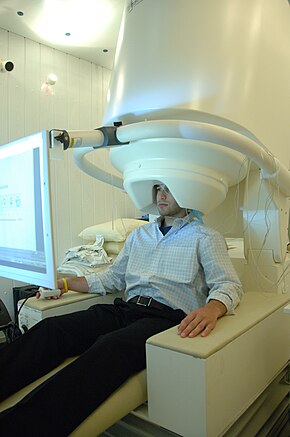 Person undergoing an 1000000 | |
| MeSH | D015225 |
Magnetoencephalography (MEG) is a functional neuroimaging technique for mapping brain activeness by recording magnetic fields produced by electrical currents occurring naturally in the encephalon, using very sensitive magnetometers. Arrays of SQUIDs (superconducting quantum interference devices) are currently the virtually mutual magnetometer, while the SERF (spin exchange relaxation-free) magnetometer is being investigated for future machines.[1] [2] Applications of MEG include basic inquiry into perceptual and cognitive brain processes, localizing regions affected by pathology before surgical removal, determining the function of diverse parts of the encephalon, and neurofeedback. This can be applied in a clinical setting to find locations of abnormalities also as in an experimental setting to simply measure brain activeness.[iii]
History [edit]

Dr. Cohen's shielded room at MIT, in which first 1000000 was measured with a SQUID

First One thousand thousand measured with SQUID, in Dr. Cohen'due south room at MIT
MEG signals were first measured by University of Illinois physicist David Cohen in 1968,[4] before the availability of the SQUID, using a copper induction coil as the detector. To reduce the magnetic background racket, the measurements were made in a magnetically shielded room. The ringlet detector was barely sensitive enough, resulting in poor, noisy Million measurements that were hard to use. Later, Cohen congenital a much better shielded room at MIT, and used ane of the first SQUID detectors, just developed by James Due east. Zimmerman, a researcher at Ford Motor Company,[5] to again measure MEG signals.[six] This time the signals were almost equally clear as those of EEG. This stimulated the interest of physicists who had been looking for uses of SQUIDs. Subsequent to this, various types of spontaneous and evoked MEGs began to exist measured.
At get-go, a single SQUID detector was used to successively measure the magnetic field at a number of points around the subject field'due south head. This was cumbersome, and, in the 1980s, MEG manufacturers began to arrange multiple sensors into arrays to cover a larger area of the head. Present-day Meg arrays are fix in a helmet-shaped vacuum flask that typically contain 300 sensors, roofing nigh of the head. In this way, MEGs of a subject or patient tin can now be accumulated quickly and efficiently.
Recent developments effort to increase portability of Million scanners by using spin exchange relaxation-costless (SERF) magnetometers. SERF magnetometers are relatively pocket-sized, equally they do non require bulky cooling systems to operate. At the same time, they feature sensitivity equivalent to that of SQUIDs. In 2012, it was demonstrated that 1000000 could work with a chip-calibration atomic magnetometer (CSAM, type of SERF).[7] More recently, in 2017, researchers congenital a working prototype that uses SERF magnetometers installed into portable individually 3D-printed helmets,[ii] which they noted in interviews could be replaced with something easier to use in future, such equally a wheel helmet.
The basis of the MEG bespeak [edit]
Synchronized neuronal currents induce weak magnetic fields. The brain's magnetic field, measuring at 10 femtotesla (fT) for cortical activity and 103 fT for the man alpha rhythm, is considerably smaller than the ambience magnetic noise in an urban environment, which is on the order of 108 fT or 0.one μT. The essential problem of biomagnetism is, thus, the weakness of the signal relative to the sensitivity of the detectors, and to the competing environmental dissonance.

Origin of the brain'south magnetic field. The electric current also produces the EEG signal.
The Million (and EEG) signals derive from the net effect of ionic currents flowing in the dendrites of neurons during synaptic transmission. In accordance with Maxwell's equations, any electrical current volition produce a magnetic field, and it is this field that is measured. The net currents can be thought of as current dipoles,[8] i.eastward. currents with a position, orientation, and magnitude, but no spatial extent[ dubious ]. According to the correct-manus rule, a electric current dipole gives rise to a magnetic field that points around the axis of its vector component.
To generate a point that is detectable, approximately fifty,000 active neurons are needed.[9] Since current dipoles must have similar orientations to generate magnetic fields that reinforce each other, information technology is often the layer of pyramidal cells, which are situated perpendicular to the cortical surface, that gives rise to measurable magnetic fields. Bundles of these neurons that are orientated tangentially to the scalp surface project measurable portions of their magnetic fields outside of the head, and these bundles are typically located in the sulci. Researchers are experimenting with various indicate processing methods in the search for methods that detect deep brain (i.east., non-cortical) signal, but no clinically useful method is currently available.
It is worth noting that activity potentials do not usually produce an appreciable field, mainly considering the currents associated with activity potentials flow in opposite directions and the magnetic fields abolish out. However, action fields take been measured from peripheral nervus system.
Magnetic shielding [edit]
Since the magnetic signals emitted by the encephalon are on the order of a few femtoteslas, shielding from external magnetic signals, including the Earth's magnetic field, is necessary. Appropriate magnetic shielding can exist obtained by constructing rooms made of aluminium and mu-metal for reducing loftier-frequency and low-frequency noise, respectively.

Archway to MSR, showing the separate shielding layers
Magnetically shielded room (MSR) [edit]
A magnetically shielded room (MSR) model consists of 3 nested main layers. Each of these layers is fabricated of a pure aluminium layer plus a high-permeability ferromagnetic layer, like in composition to molybdenum permalloy. The ferromagnetic layer is supplied as i mm sheets, while the innermost layer is composed of 4 sheets in close contact, and the outer 2 layers are composed of 3 sheets each. Magnetic continuity is maintained by overlay strips. Insulating washers are used in the spiral assemblies to ensure that each master layer is electrically isolated. This helps eliminate radio frequency radiation, which would dethrone SQUID performance. Electrical continuity of the aluminium is also maintained by aluminium overlay strips to ensure Ac eddy electric current shielding, which is of import at frequencies greater than i Hz. The junctions of the inner layer are often electroplated with silver or gold to meliorate conductivity of the aluminium layers.[10]
Active shielding organization [edit]
Agile systems are designed for three-dimensional racket counterfoil. To implement an active system, low-noise fluxgate magnetometers are mounted at the center of each surface and oriented orthogonally to it. This negatively feeds a DC amplifier through a low-laissez passer network with a slow falloff to minimize positive feedback and oscillation. Congenital into the organization are shaking and degaussing wires. Shaking wires increment the magnetic permeability, while the permanent degaussing wires are applied to all surfaces of the inner main layer to degauss the surfaces.[4] Moreover, noise cancellation algorithms can reduce both depression-frequency and loftier-frequency dissonance. Mod systems take a noise floor of around ii–3 fT/Hz0.5 above 1 Hz.
Source localization [edit]
The inverse problem [edit]
The challenge posed by MEG is to make up one's mind the location of electric activeness inside the brain from the induced magnetic fields outside the head. Problems such equally this, where model parameters (the location of the activity) have to be estimated from measured data (the SQUID signals) are referred to as inverse problems (in contrast to forward bug [xi] where the model parameters (e.g. source location) are known and the data (due east.k. the field at a given distance) is to be estimated.) The primary difficulty is that the inverse problem does not have a unique solution (i.e., there are space possible "correct" answers), and the trouble of defining the "best" solution is itself the subject of intensive research.[12] Possible solutions tin can exist derived using models involving prior knowledge of brain activity.
The source models tin exist either over-determined or under-determined. An over-adamant model may consist of a few point-like sources ("equivalent dipoles"), whose locations are so estimated from the data. Under-determined models may be used in cases where many different distributed areas are activated ("distributed source solutions"): at that place are infinitely many possible current distributions explaining the measurement results, but the most probable is selected. Localization algorithms make use of given source and head models to discover a likely location for an underlying focal field generator.
Ane type of localization algorithm for overdetermined models operates by expectation-maximization: the system is initialized with a first estimate. A loop is started, in which a forrad model is used to simulate the magnetic field that would consequence from the current guess. The estimate is adjusted to reduce the discrepancy between the fake field and the measured field. This process is iterated until convergence.
Some other common technique is beamforming, wherein a theoretical model of the magnetic field produced by a given current dipole is used equally a prior, forth with second-order statistics of the data in the form of a covariance matrix, to calculate a linear weighting of the sensor array (the beamformer) via the Backus-Gilbert inverse. This is too known as a linearly constrained minimum variance (LCMV) beamformer. When the beamformer is applied to the data, it produces an judge of the power in a "virtual channel" at the source location.
The extent to which the constraint-free MEG inverse problem is sick-posed cannot be overemphasized. If one'due south goal is to estimate the electric current density within the human brain with say a 5mm resolution so it is well established that the vast majority of the data needed to perform a unique inversion must come not from the magnetic field measurement but rather from the constraints applied to the problem. Furthermore, fifty-fifty when a unique inversion is possible in the presence of such constraints said inversion tin exist unstable. These conclusions are easily deduced from published works.[13]
Magnetic source imaging [edit]
The source locations can be combined with magnetic resonance imaging (MRI) images to create magnetic source images (MSI). The two sets of data are combined by measuring the location of a common gear up of fiducial points marked during MRI with lipid markers and marked during One thousand thousand with electrified coils of wire that give off magnetic fields. The locations of the fiducial points in each data prepare are so used to ascertain a common coordinate system so that superimposing the functional MEG data onto the structural MRI data ("coregistration") is possible.
A criticism of the use of this technique in clinical exercise is that it produces colored areas with definite boundaries superimposed upon an MRI scan: the untrained viewer may not realize that the colors do not represent a physiological certainty, non because of the relatively low spatial resolution of MEG, but rather some inherent uncertainty in the probability cloud derived from statistical processes. However, when the magnetic source image corroborates other data, information technology can be of clinical utility.
Dipole model source localization [edit]
A widely accepted source-modeling technique for MEG involves computing a set of equivalent current dipoles (ECDs), which assumes the underlying neuronal sources to exist focal. This dipole fitting procedure is not-linear and over-determined, since the number of unknown dipole parameters is smaller than the number of One thousand thousand measurements.[fourteen] Automated multiple dipole model algorithms such as multiple signal classification (MUSIC) and multi-start spatial and temporal modeling (MSST) are applied to the analysis of Million responses. The limitations of dipole models for characterizing neuronal responses are (1) difficulties in localizing extended sources with ECDs, (2) bug with accurately estimating the total number of dipoles in advance, and (3) dependency on dipole location, especially depth in the brain.
Distributed source models [edit]
Different multiple-dipole modeling, distributed source models divide the source space into a filigree containing a large number of dipoles. The inverse trouble is to obtain the dipole moments for the grid nodes.[15] Every bit the number of unknown dipole moments is much greater than the number of Meg sensors, the inverse solution is highly underdetermined, and so additional constraints are needed to reduce ambiguity of the solution. The main reward of this approach is that no prior specification of the source model is necessary. However, the resulting distributions may exist hard to interpret, because they only reflect a "blurred" (or even distorted) image of the true neuronal source distribution. The matter is complicated by the fact that spatial resolution depends strongly on several parameters such as brain area, depth, orientation, number of sensors etc.[sixteen]
Independent component analysis (ICA) [edit]
Contained component analysis (ICA) is another point processing solution that separates different signals that are statistically independent in time. It is primarily used to remove artifacts such as blinking, eye musculus move, facial muscle artifacts, cardiac artifacts, etc. from Million and EEG signals that may be contaminated with outside noise.[17] Still, ICA has poor resolution of highly correlated brain sources.
Use in the field [edit]
In inquiry, Million's primary use is the measurement of time courses of action. 1000000 can resolve events with a precision of 10 milliseconds or faster, while functional magnetic resonance imaging (fMRI), which depends on changes in blood flow, can at best resolve events with a precision of several hundred milliseconds. MEG also accurately pinpoints sources in primary auditory, somatosensory, and motor areas. For creating functional maps of human cortex during more than circuitous cognitive tasks, MEG is most ofttimes combined with fMRI, as the methods complement each other. Neuronal (MEG) and hemodynamic fMRI data do not necessarily agree, in spite of the tight relationship between local field potentials (LFP) and blood oxygenation level-dependent (BOLD) signals. MEG and BOLD signals may originate from the same source (though the BOLD signals are filtered through the hemodynamic response).
MEG is also being used to better localize responses in the brain. The openness of the MEG setup allows external auditory and visual stimuli to be easily introduced. Some movement by the subject is also possible as long equally it does non jar the subject's head. The responses in the brain before, during, and afterwards the introduction of such stimuli/motion can and so be mapped with greater spatial resolution than was previously possible with EEG.[18] Psychologists are besides taking advantage of MEG neuroimaging to better understand relationships between encephalon office and behavior. For case, a number of studies have been done comparing the MEG responses of patients with psychological troubles to control patients. There has been smashing success isolating unique responses in patients with schizophrenia, such as auditory gating deficits to human voices.[xix] MEG is also being used to correlate standard psychological responses, such as the emotional dependence of language comprehension.[20]
Contempo studies have reported successful classification of patients with multiple sclerosis, Alzheimer's illness, schizophrenia, Sjögren's syndrome, chronic alcoholism, facial pain and thalamocortical dysrhythmias. One thousand thousand tin be used to distinguish these patients from healthy control subjects, suggesting a time to come role of One thousand thousand in diagnostics.[21] [22]
A large office of the difficulty and cost of using MEG is the need for manual analysis of the information. Progress has been made in analysis by computer, comparing a patient's scans with those drawn from a big database of normal scans, with the potential to reduce cost greatly.[23]
Brain connectivity and neural oscillations [edit]
Based on its perfect temporal resolution, magnetoencephalography (1000000) is now heavily used to study oscillatory activeness in the encephalon, both in terms of local neural synchrony and cantankerous-area synchronisation. As an example for local neural synchrony, MEG has been used to investigate alpha rhythms in various targeted brain regions, such as in visual[24] [25] or auditory cortex.[26] Other studies have used MEG to study the neural interactions betwixt different brain regions (e.yard., between frontal cortex and visual cortex).[27] Magnetoencephalography can also be used to report changes in neural oscillations across unlike stages of consciousness, such as in sleep.[28]
Focal epilepsy [edit]
The clinical uses of Meg are in detecting and localizing pathological activity in patients with epilepsy, and in localizing eloquent cortex for surgical planning in patients with brain tumors or intractable epilepsy. The goal of epilepsy surgery is to remove the epileptogenic tissue while sparing salubrious brain areas.[29] Knowing the exact position of essential brain regions (such equally the primary motor cortex and primary sensory cortex, visual cortex, and areas involved in spoken language production and comprehension) helps to avert surgically induced neurological deficits. Direct cortical stimulation and somatosensory evoked potentials recorded on electrocorticography (ECoG) are considered the aureate standard for localizing essential brain regions. These procedures can be performed either intraoperatively or from chronically indwelling subdural grid electrodes. Both are invasive.
Noninvasive Meg localizations of the central sulcus obtained from somatosensory evoked magnetic fields testify strong agreement with these invasive recordings.[30] [31] [32] One thousand thousand studies assist in clarification of the functional arrangement of primary somatosensory cortex and to delineate the spatial extent of hand somatosensory cortex past stimulation of the individual digits. This agreement between invasive localization of cortical tissue and 1000000 recordings shows the effectiveness of MEG analysis and indicates that Million may substitute invasive procedures in the future.
Fetal [edit]
1000000 has been used to report cerebral processes such as vision, audition, and language processing in fetuses and newborns.[33]
Traumatic encephalon injury [edit]
One thousand thousand can exist used to identify traumatic brain injury, which is especially common among soldiers exposed to explosions. Such injuries are not easily diagnosed by other methods, and are oftentimes misdiagnosed as mail service-traumatic stress disorder (PTSD).[23]
[edit]
MEG has been in evolution since the 1960s only has been greatly aided by recent advances in computing algorithms and hardware, and promises improved spatial resolution coupled with extremely high temporal resolution (better than i ms). Since the MEG signal is a directly measure out of neuronal activity, its temporal resolution is comparable with that of intracranial electrodes.
MEG complements other brain activeness measurement techniques such as electroencephalography (EEG), positron emission tomography (PET), and fMRI. Its strengths consist in independence of head geometry compared to EEG (unless ferromagnetic implants are present), non-invasiveness, use of no ionizing radiations, as opposed to PET and high temporal resolution equally opposed to fMRI.
Million in comparison to EEG [edit]
Although EEG and MEG signals originate from the same neurophysiological processes, there are important differences.[34] Magnetic fields are less distorted than electrical fields by the skull and scalp, which results in a better spatial resolution of the MEG. Whereas scalp EEG is sensitive to both tangential and radial components of a current source in a spherical volume conductor, One thousand thousand detects only its tangential components. Scalp EEG can, therefore, detect activity both in the sulci and at the top of the cortical gyri, whereas MEG is most sensitive to activity originating in sulci. EEG is, therefore, sensitive to action in more than brain areas, but activity that is visible in Meg tin can also be localized with more accuracy.
Scalp EEG is sensitive to extracellular volume currents produced by postsynaptic potentials. MEG detects intracellular currents associated primarily with these synaptic potentials because the field components generated by book currents tend to cancel out in a spherical volume usher.[35] The decay of magnetic fields every bit a part of distance is more than pronounced than for electric fields. Therefore, MEG is more sensitive to superficial cortical activity, which makes it useful for the study of neocortical epilepsy. Finally, MEG is reference-free, while scalp EEG relies on a reference that, when active, makes estimation of the data difficult.
See also [edit]
- Auditory evoked field
- Direct encephalon interfaces
- Electrophysiology
- Evoked field
- FieldTrip
- Magnetocardiography
- Magnetogastrography
- Magnetometer
- Magnetomyography
- SQUID
- Whole encephalon emulation
References [edit]
- ^ Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV (1993). "Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working homo brain" (PDF). Reviews of Modern Physics. 65 (2): 413–497. Bibcode:1993RvMP...65..413H. doi:10.1103/RevModPhys.65.413. ISSN 0034-6861.
- ^ a b Boto, Elena; Holmes, Niall; Leggett, James; Roberts, Gillian; Shah, Vishal; Meyer, Sofie Southward.; Muñoz, Leonardo Duque; Mullinger, Karen J.; Tierney, Tim M. (March 2018). "Moving magnetoencephalography towards real-world applications with a habiliment system". Nature. 555 (7698): 657–661. Bibcode:2018Natur.555..657B. doi:x.1038/nature26147. ISSN 1476-4687. PMC6063354. PMID 29562238.
- ^ Carlson NR (2013). Physiology of Behavior . Upper Saddle River, NJ: Pearson Education Inc. pp. 152–153. ISBN978-0-205-23939-nine.
- ^ a b Cohen D (August 1968). "Magnetoencephalography: evidence of magnetic fields produced by alpha-rhythm currents". Scientific discipline. 161 (3843): 784–6. Bibcode:1968Sci...161..784C. doi:10.1126/scientific discipline.161.3843.784. PMID 5663803. S2CID 34001253.
- ^ Zimmerman JE, Theine P, Harding JT (1970). "Design and operation of stable rf-biased superconducting point-contact breakthrough devices, etc". Periodical of Applied Physics. 41 (4): 1572–1580. doi:10.1063/ane.1659074.
- ^ Cohen D (February 1972). "Magnetoencephalography: detection of the brain'southward electrical activity with a superconducting magnetometer" (PDF). Science. 175 (4022): 664–half-dozen. Bibcode:1972Sci...175..664C. doi:10.1126/science.175.4022.664. PMID 5009769. S2CID 29638065.
- ^ Sander TH, Preusser J, Mhaskar R, Kitching J, Trahms L, Knappe S (May 2012). "Magnetoencephalography with a chip-scale atomic magnetometer". Biomedical Eyes Express. iii (5): 981–90. doi:10.1364/BOE.3.000981. PMC3342203. PMID 22567591.
- ^ Hämäläinen, Matti; Hari, Riitta; Ilmoniemi, Risto J.; Knuutila, Jukka; Lounasmaa, Olli V. (1993-04-01). "Magnetoencephalography---theory, instrumentation, and applications to noninvasive studies of the working human brain". Reviews of Mod Physics. 65 (ii): 413–497. Bibcode:1993RvMP...65..413H. doi:10.1103/RevModPhys.65.413.
- ^ Okada Y (1983). "Neurogenesis of evoked magnetic fields". In Williamson SH, Romani GL, Kaufman L, Modena I (eds.). Biomagnetism: an Interdisciplinary Approach. New York: Plenum Press. pp. 399–408. ISBN978-1-4757-1785-3.
- ^ Cohen D, Schläpfer U, Ahlfors S, Hämäläinen Yard, Halgren Eastward. "New Six-Layer Magnetically-Shielded Room for MEG" (PDF). Charlestown, Massachusetts: Athinoula A. Martinos Heart for Biomedical Imaging, Massachusetts Full general Infirmary. S2CID 27016664. Archived from the original (PDF) on 2020-08-03.
- ^ Tanzer IO (2006). Numerical Modeling in Electro- and Magnetoencephalography (Ph.D. thesis). Finland: Helsinki Academy of Technology.
- ^ Hauk O, Wakeman DG, Henson R (February 2011). "Comparison of racket-normalized minimum norm estimates for MEG analysis using multiple resolution metrics". NeuroImage. 54 (3): 1966–74. doi:10.1016/j.neuroimage.2010.09.053. PMC3018574. PMID 20884360.
- ^ Sheltraw D, Coutsias East (2003). "Invertibility of current density from near-field electromagnetic information" (PDF). Periodical of Practical Physics. 94 (8): 5307–5315. Bibcode:2003JAP....94.5307S. doi:10.1063/1.1611262.
- ^ Huang MX, Dale AM, Song T, Halgren Due east, Harrington DL, Podgorny I, Canive JM, Lewis Due south, Lee RR (July 2006). "Vector-based spatial-temporal minimum L1-norm solution for MEG". NeuroImage. 31 (3): 1025–37. doi:x.1016/j.neuroimage.2006.01.029. PMID 16542857. S2CID 9607000.
- ^ Hämäläinen MS, Ilmoniemi RJ (January 1994). "Interpreting magnetic fields of the brain: minimum norm estimates". Medical & Biological Applied science & Computing. 32 (1): 35–42. doi:ten.1007/BF02512476. PMID 8182960. S2CID 6796187.
- ^ Molins A, Stufflebeam SM, Brown EN, Hämäläinen MS (September 2008). "Quantification of the benefit from integrating 1000000 and EEG data in minimum l2-norm estimation". NeuroImage. 42 (3): 1069–77. doi:10.1016/j.neuroimage.2008.05.064. PMID 18602485. S2CID 6462818.
- ^ Jung TP, Makeig South, Westerfield K, Townsend J, Courchesne Eastward, Sejnowski TJ (Oct 2000). "Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects". Clinical Neurophysiology. 111 (10): 1745–58. CiteSeerX10.one.1.164.9941. doi:10.1016/S1388-2457(00)00386-two. PMID 11018488. S2CID 11044416.
- ^ Cui R, Cunnington R, Beisteiner R, Deecke Fifty (2012). "Furnishings of force-load on cortical action preceding voluntary finger movement". Neurology, Psychiatry and Brain Research. 18 (3): 97–104. doi:ten.1016/j.npbr.2012.03.001.
- ^ Hirano Y, Hirano S, Maekawa T, Obayashi C, Oribe N, Monji A, Kasai K, Kanba South, Onitsuka T (March 2010). "Auditory gating deficit to human voices in schizophrenia: a One thousand thousand study". Schizophrenia Inquiry. 117 (ane): 61–7. doi:10.1016/j.schres.2009.09.003. PMID 19783406. S2CID 7845180.
- ^ Ihara A, Wei Q, Matani A, Fujimaki N, Yagura H, Nogai T, Umehara H, Murata T (Jan 2012). "Linguistic communication comprehension dependent on emotional context: a magnetoencephalography written report". Neuroscience Inquiry. 72 (1): 50–8. doi:10.1016/j.neures.2011.09.011. PMID 22001763. S2CID 836242.
- ^ Georgopoulos AP, Karageorgiou Due east, Leuthold AC, Lewis SM, Lynch JK, Alonso AA, Aslam Z, Carpenter AF, Georgopoulos A, Hemmy LS, Koutlas IG, Langheim FJ, McCarten JR, McPherson SE, Pardo JV, Pardo PJ, Parry GJ, Rottunda SJ, Segal BM, Sponheim SR, Stanwyck JJ, Stephane M, Westermeyer JJ (Dec 2007). "Synchronous neural interactions assessed by magnetoencephalography: a functional biomarker for brain disorders". Journal of Neural Applied science. 4 (iv): 349–55. Bibcode:2007JNEng...4..349G. doi:10.1088/1741-2560/4/4/001. hdl:10161/12446. PMID 18057502.
- ^ Montez T, Poil SS, Jones BF, Manshanden I, Verbunt JP, van Dijk BW, Brussaard AB, van Ooyen A, Stam CJ, Scheltens P, Linkenkaer-Hansen K (Feb 2009). "Altered temporal correlations in parietal blastoff and prefrontal theta oscillations in early-stage Alzheimer affliction". Proceedings of the National Academy of Sciences of the United States of America. 106 (five): 1614–nine. Bibcode:2009PNAS..106.1614M. doi:ten.1073/pnas.0811699106. PMC2635782. PMID 19164579.
- ^ a b Rose, David (twenty February 2022). "British army veterans denied treatment for encephalon injuries". The Observer.
- ^ Bagherzadeh, Yasaman; Baldauf, Daniel; Pantazis, Dimitrios; Desimone, Robert (February 2020). "Alpha Synchrony and the Neurofeedback Control of Spatial Attention". Neuron. 105 (3): 577–587.e5. doi:10.1016/j.neuron.2019.11.001. ISSN 0896-6273.
- ^ de Vries, Eelke; Baldauf, Daniel (2019-x-01). "Attentional Weighting in the Confront Processing Network: A Magnetic Response Prototype-guided Magnetoencephalography Written report Using Multiple Cyclic Entrainments". Journal of Cerebral Neuroscience. 31 (10): 1573–1588. doi:10.1162/jocn_a_01428. ISSN 0898-929X.
- ^ Vries, Ingmar Eastward. J. de; Marinato, Giorgio; Baldauf, Daniel (2021-08-24). "Decoding object-based auditory attention from source-reconstructed One thousand thousand alpha oscillations". Journal of Neuroscience. doi:10.1523/JNEUROSCI.0583-21.2021. ISSN 0270-6474. PMC8513695. PMID 34429378.
- ^ Baldauf, D.; Desimone, R. (2014-04-25). "Neural Mechanisms of Object-Based Attention". Science. 344 (6182): 424–427. doi:10.1126/science.1247003. ISSN 0036-8075.
- ^ Brancaccio, Arianna; Tabarelli, Davide; Bigica, Marco; Baldauf, Daniel (2020-04-24). "Cortical source localization of sleep-stage specific oscillatory activeness". Scientific Reports. x (1): 6976. doi:x.1038/s41598-020-63933-v. ISSN 2045-2322. PMC7181624. PMID 32332806.
- ^ Luders HO (1992). Epilepsy Surgery. New York Raven Press.
- ^ Sutherling WW, Crandall PH, Darcey TM, Becker DP, Levesque MF, Barth DS (November 1988). "The magnetic and electric fields concord with intracranial localizations of somatosensory cortex". Neurology. 38 (eleven): 1705–14. doi:10.1212/WNL.38.11.1705. PMID 3185905. S2CID 8828767.
- ^ Rowley HA, Roberts TP (Nov 1995). "Functional localization by magnetoencephalography". Neuroimaging Clinics of North America. v (4): 695–710. PMID 8564291.
- ^ Gallen CC, Hirschkoff EC, Buchanan DS (May 1995). "Magnetoencephalography and magnetic source imaging. Capabilities and limitations". Neuroimaging Clinics of North America. 5 (2): 227–49. PMID 7640886.
- ^ Sheridan CJ, Matuz T, Draganova R, Eswaran H, Preissl H (2010). "Fetal Magnetoencephalography - Achievements and Challenges in the Study of Prenatal and Early on Postnatal Brain Responses: A Review". Infant and Child Development. 19 (1): eighty–93. doi:x.1002/icd.657. PMC2830651. PMID 20209112.
- ^ Cohen D, Cuffin BN (July 1983). "Demonstration of useful differences between magnetoencephalogram and electroencephalogram". Electroencephalography and Clinical Neurophysiology. 56 (1): 38–51. doi:10.1016/0013-4694(83)90005-6. PMID 6190632.
- ^ Barth DS, Sutherling W, Beatty J (March 1986). "Intracellular currents of interictal penicillin spikes: evidence from neuromagnetic mapping". Brain Research. 368 (1): 36–48. doi:ten.1016/0006-8993(86)91040-1. PMID 3955364. S2CID 3078690.
Farther reading [edit]
- Baillet Southward, Mosher JC, Leahy RM (November 2001). "Electromagnetic Brain Mapping". IEEE Signal Processing Magazine. 18 (6): fourteen–thirty. Bibcode:2001ISPM...18...14B. doi:x.1109/79.962275.
- Cohen D (2004). "Boston and the history of biomagnetism". Neurology and Clinical Neurophysiology. thirty (1): 114. PMID 16012683.
- Cohen D, Halgren E (2004). "Magnetoencephalography". In Adelman G, Smith B (eds.). Encyclopedia of Neuroscience. Elsevier.
- Hämäläinen Thousand, Hari R, Ilmoniemi R, Knuutila J, Lounasmaa OV (1993). "Magnetoencephalography – theory, instrumentation, and applications to noninvasive studies of bespeak processing in the human encephalon" (PDF). Reviews of Modernistic Physics. 65 (2): 413–497. Bibcode:1993RvMP...65..413H. doi:ten.1103/revmodphys.65.413.
- Hansen PC, Kringelbach ML, Salmelin R (2010). MEG: An Introduction to Methods. New York: Oxford University Printing Inc.
- Murakami S, Okada Y (September 2006). "Contributions of primary neocortical neurons to magnetoencephalography and electroencephalography signals". The Journal of Physiology. 575 (Pt iii): 925–36. doi:10.1113/jphysiol.2006.105379. PMC1995687. PMID 16613883.
- Suk J, Ribary U, Cappell J, Yamamoto T, Llinás R (March 1991). "Anatomical localization revealed by MEG recordings of the human somatosensory arrangement". Electroencephalography and Clinical Neurophysiology. 78 (iii): 185–96. doi:10.1016/0013-4694(91)90032-y. PMID 1707790.
- Tanzer OI (2006). Numerical Modeling in Electro- and Magnetoencephalography (Ph.D. thesis). Finland: Helsinki Academy of Engineering.
- Wolters CH, Anwander A, Tricoche X, Weinstein D, Koch MA, MacLeod RS (2006). "Influence of Tissue Conductivity Anisotropy on EEG/MEG Field and Return Current Computation in a realistic Caput Model: A Simulation and Visualization Written report using High-Resolution Finite Element Modeling". NeuroImage. 30 (three): 813–826. doi:10.1016/j.neuroimage.2005.10.014. hdl:11858/00-001M-0000-0010-BD20-9. PMID 16364662. S2CID 5578998.
Which Two Of The Following Reduce The Background Noise In Magneto- Medicine?,
Source: https://en.wikipedia.org/wiki/Magnetoencephalography
Posted by: hughesbegadd.blogspot.com


0 Response to "Which Two Of The Following Reduce The Background Noise In Magneto- Medicine?"
Post a Comment